Higher-Order Cycloadditions
Introduction
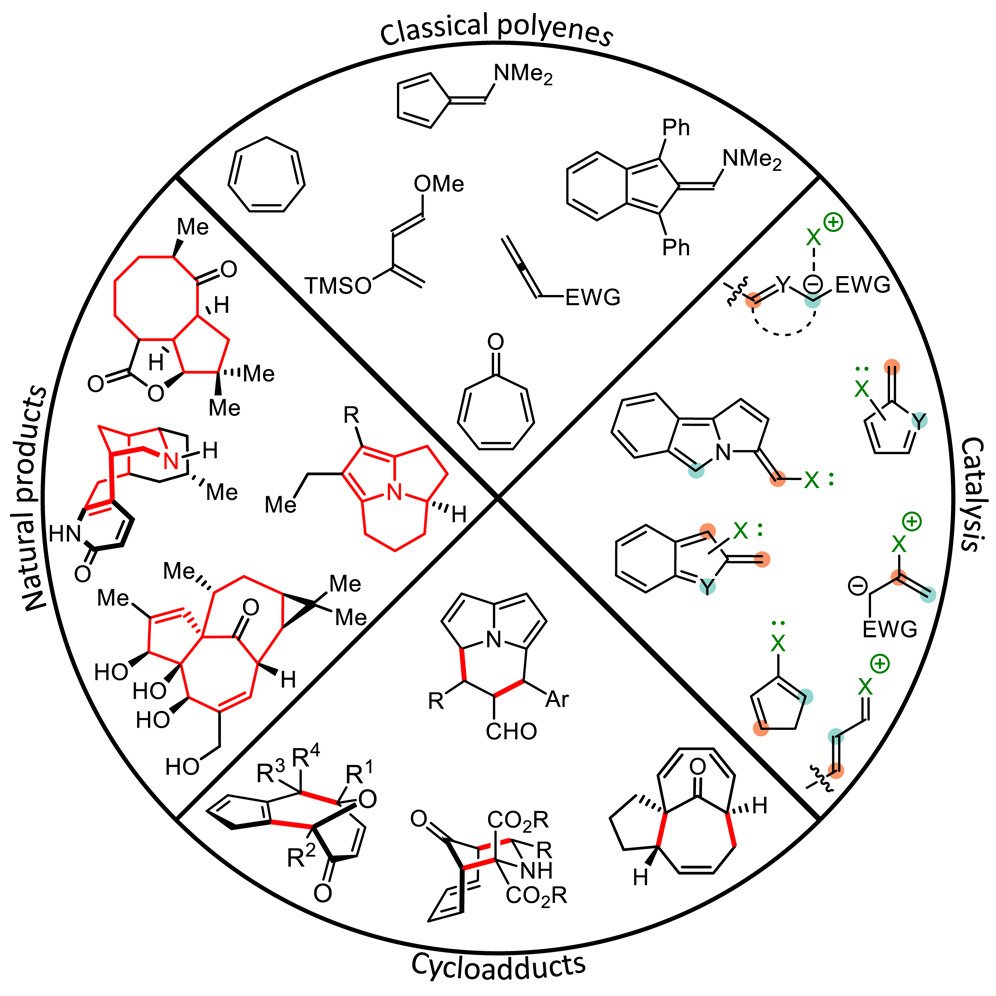
The introduction of orbital symmetry selection rules for cycloadditions in 1965 classified these reactions in terms of electronic characteristics and opened a new way of understanding reactions and looking for new ones. Cycloadditions involving 6π-electrons (or fewer), such as the Diels-Alder reaction, are thoroughly investigated reactions, which follow the Woodward-Hoffmann rules. Medium-sized rings, particularly those embedded within complex polycyclic natural products, are not easily accessed due to a dearth of synthetic methods. Thus, cycloadditions with >6π-electrons—higher-order cycloadditions (HOCs)—have proven to be strategic disconnections for the rapid and efficient synthesis of complex ring systems. Despite the introduction of the Woodward−Hoffmann rules, several examples have illustrated over the years the difficulty in predicting which of the possible transformations will occur when two highly unsaturated molecules react. Historically, HOCs have been plagued by low yields, poor periselectivity and the development of enantioselective variants has been elusive.
The rise of organocatalysis in asymmetric synthesis has led to a surge of interest in stereoselective versions of HOCs as novel reactive intermediates are now accessible with the opportunity for exceptional stereoselectivity. Traditionally, HOCs have relied on the stoichiometric formation of highly reactive intermediates, reaction of these classical intermediates can be difficult to control—they are typically air/moisture sensitive and, as a result, difficult to isolate. Catalytic generation of reactive intermediates therefore offers distinct advantages. Most of the organocatalytic strategies developed thus far leverage stable aldehyde precursors, which, upon activation by a catalyst, form the required reactive cycloaddend. Upon catalytic formation of the active cycloaddend, its subsequent participation in HOCs may proceed in a highly controlled manner.
Our work
In 2017 we utilized cinchona alkaloid-derived aminocatalysts for the activation of 2-cycloalkenones in the first examples of enantioselective intermolecular [6 + 4]- and [6 + 2]- cycloadditions. Prior to this, only a few enantioselective higher-order cycloadditions had been reported. Since then, we have developed several organocatalytic approaches (mostly relying on enamine catalysis), to carry out HOCs, including the first catalytic enantioselective examples of a [10 + 4]- and a [12 + 2]- cycloaddition. In addition, organocatalytic hetero-[6 + 4]- and [6 + 2]- cycloadditions have been conducted, providing rapid access to the biologically relevant pyrrolo-azepine core that proceed for a series of formyl substituted heteroaromatic compounds such as pyrroles, imidazoles, and pyrazoles. More recently, we have demonstrated that aminocatalyzed HOCs can be used to access the tricyclic cycl[3.2.2]azine core with a ring-junction nitrogen atom, which are abundant in naturally occurring alkaloids.
A remarkable aspect of these recent developments is the cross-disciplinary research involved: from organic synthesis to computational studies in cooperation with the Houk group integrated with experimental studies of reaction mechanisms, intermediates, and dynamics. While the Woodward−Hoffmann rules strictly only apply to concerted processes, the orbital interactions causing a reaction to be orbital symmetry-allowed may be maintained along a dynamically stepwise pathway, leading only to allowed products. Most HOCs display a pronounced asynchronicity in the transition state (TS) structure and exhibit both dynamically concerted and stepwise trajectories, sometimes even fully stepwise processes. Interestingly, in several instances computations have shown that HOCs proceed through bis-pericyclic, or more generally ambimodal, TSs. Such a TS can lead to multiple products without intervening minima or secondary barriers because the reaction path bifurcates after the TS.
A survey of the currently known organocatalytic HOCs reveals a dependency on a small subset of recurring motifs: penta- and heptafulvenoids. The development of alternative activation modes to access novel cycloaddends may permit the usage of handles beyond aldehyde precursors for catalytic activation, as well as chiral induction, and ultimately broaden the pool of applicable substrates for HOCs. Merging HOCs with currently under-utilized catalytic strategies, such as noncovalent catalysis or activation of heptafulvenes, may require more than simple adaptation of current organocatalytic strategies. Finally, cycloadditions relying on organocatalytically generated cycloaddends containing >10π-electrons are currently exceedingly rare and would thus offer interesting prospects to further develop the field.
Selected Articles on Higher-Order Cycloadditions
Cycloaddition Reactions: Why Is It So Challenging To Move from Six to Ten Electrons?
Angew. Chem. Int. Ed. 56 (2017) 10033
Higher-Order Cycloadditions in the Age of
Catalysis.
Chem. 8 (2022) 20.
Expanding the Frontiers of Higher-Order
Cycloadditions.
Acc. Chem. Res. 52 (2019) 17856.